LiFePO4
2020-01-17 09:27:52
Natural lithium iron phosphate exists in nature and is called Triphylite. There are two types of PO4 tetrahedron and FeO6 octahedron in cubic olivine lithium iron phosphate. Fe is separated by phosphate. Each octahedron and other tetrahedrons share edges in the c-axis direction and share two angles with the other two tetrahedrons. The distance between Li-O in LiO6 octahedron is different. In the range of 0.207 ~ 0.223nm, the cross-sectional area of the one-dimensional diffusion channel of lithium ion is 0.075 ~ 0.076nm2. There is no continuous FeO; and the PO4 network, the diffusion channel is one-dimensional; there is no direct Fe-Fe interaction, so the electronic conductivity and ion diffusion coefficient are very small, respectively 10-9S / cm (even smaller) And 10-13 ~ 10-11cm2 / s. The strong P—O bond in the structure hinders the release of oxygen atoms during overcharge, and gives the material strong stability. After the lithium ions were released, the a and 6 parameters decreased, the c parameter increased slightly, the volume decreased by 6.8%, and the density increased by 2.59%. LiFePO4, space group is Pbnm, unit cell parameters are a = 0.6008nm, b = 1.0334nm, c = 0.4693nm, V = 0.2914nm3; FePO4, space group is Pbnm, unit cell parameters are a = 0.5792nm, b = 0.9821 nm, c = 0.4788nm, V = 0.2734nm3, the structure is shown in Figure 2.1.
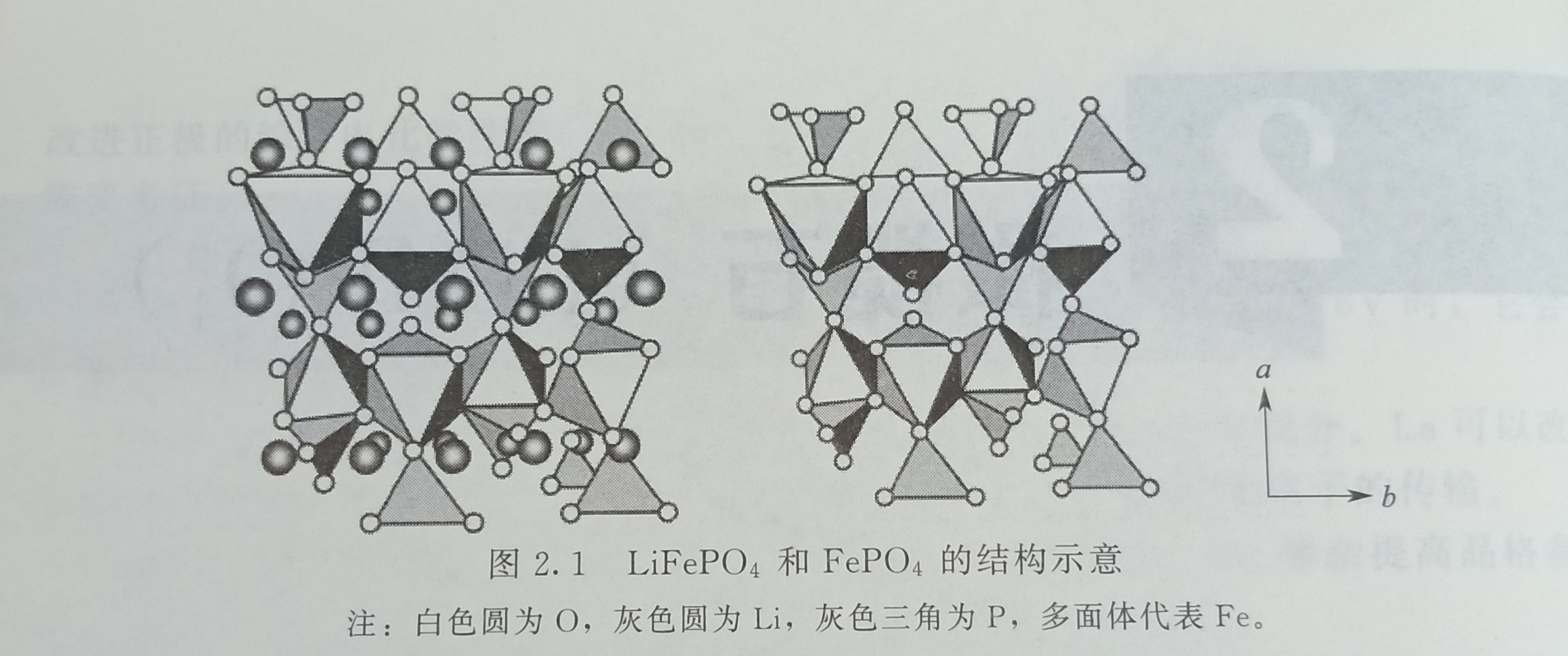
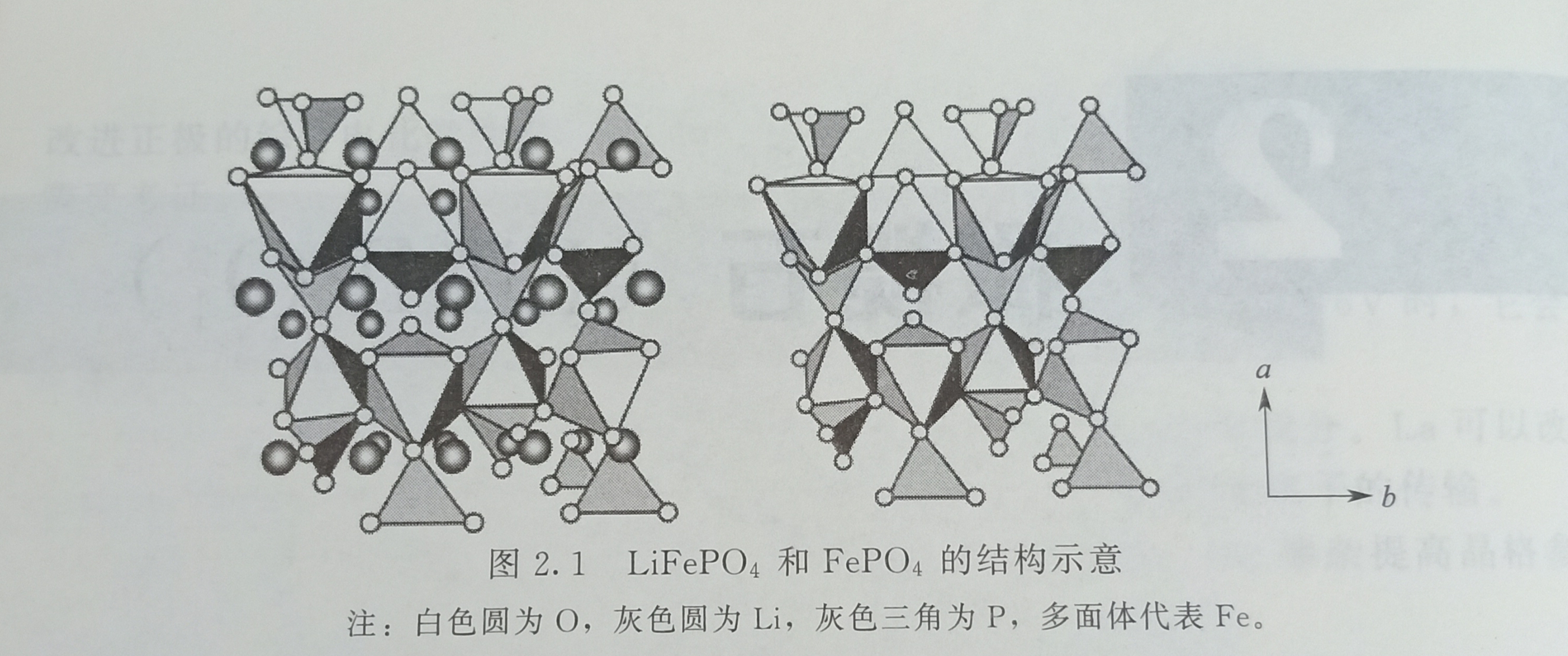
In the synthesis of LiFePO4, if lithium is insufficient, FexP2O7 impurities will be formed. In addition, there may also be F2P and Fe75P15C10. Sometimes, the actual composition of some LiFePO4 with excellent high rate performance may be LiFe1-2XP1-XO4-y.
The charge-discharge curve of LiFePO4 is shown in Figure 2.2. The first-principles calculations show that LiFePO4 is a semiconductor with a band gap of 0.27 ~ 0.3eV. The lithium desorption process is a two-phase reaction mechanism. During the lithium removal process, FePO4 is formed, and the electrochemical reaction is
LiFePO4-xLi + -xe⇄ (1-x) LiFePO4 + xFePO4 The details can be further subdivided as follows
LiFePO4→LiaFePO4 Li e
LiαFePO4⇄(1-x)LiαFePO4 Fe1-βPO4 Li e
-
skype
Zale Zhou
