活性电极材料
2020-02-17 15:17:09
Lithium electroactive electrode materials can be roughly divided into two categories according to the charge-discharge mechanism. The first type is the embedding reaction mechanism. The main structure of this type of material remains basically unchanged during the charge-discharge process. The relationship between the parent material and lithium ions is similar to the relationship between the building and the person in the building. , Spinel lithium manganate, etc .; a type of active electrode material represented by LiFePO4, its charge and discharge mechanism is different from carbon-based, spinel lithium manganate and other materials, with the formation of two phases, two phases are formed simultaneously The interface, similar to the case of ice-water mixture, strictly speaking, its charge-discharge mechanism is not a mechanism for deintercalating lithium. The second type is the conversion reaction mechanism. During the reaction, the structure of the parent phase, phase destruction and the formation of new phases are included. Such materials include Si, Sn and their oxides, and most metal oxides.
At present, the main secondary battery systems on the market include lead-acid batteries, Ni-MH batteries, and lithium-ion batteries. These three types of batteries each have their own advantages and each occupy its own application market.
The advantages and disadvantages of the three batteries are obvious, basically showing the characteristics of "your disadvantage is exactly my advantage". From the perspective of energy density and specific energy, lithium-ion batteries have obvious advantages. As shown in Figure 1.1, when used as an electric vehicle power battery, the specific energy parameter determines the driving distance, the specific power parameter determines the driving speed, and the energy density parameter determines the vehicle freedom. size of space.
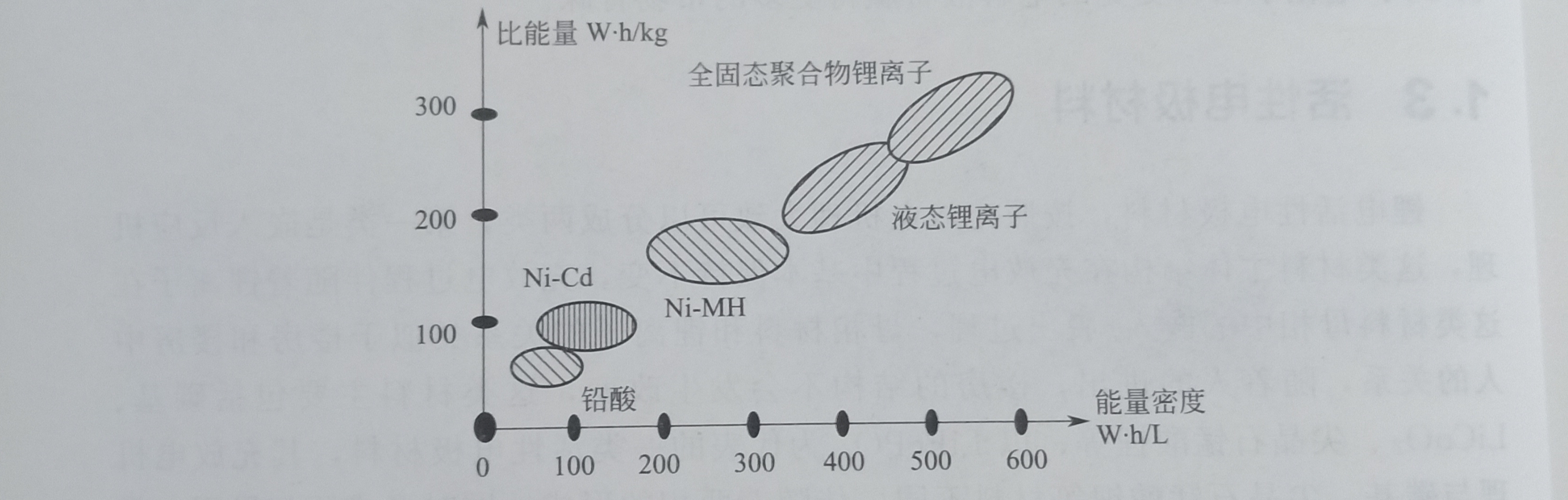
Figure 1.1 Energy comparison of different battery systems
There is a point in the business world that lithium ion may replace lead-acid batteries in the field of battery startup for automobiles in the future, which has led many lead-acid battery companies to begin voting transformation. The application of lead-acid batteries mainly includes three aspects: car starter batteries (mainstream products are 12V-20A.h and 24V-40A.h), electric bicycle batteries and backup power. The working mode of the automobile start battery is pulse discharge. The instantaneous pulse current is large and the voltage will be reduced by more than 50%. This mode is not suitable for lithium ion batteries. Therefore, I believe that lithium ion batteries cannot replace lead-acid batteries in the field of automobile startup batteries. Compared to the total weight of the car, the weight ratio of starting lead-acid batteries is very low. Even if it is replaced by a lithium-ion battery, the weight of the vehicle will not be significantly reduced. Acid batteries have become an inevitable trend. After using lithium-ion batteries, the total weight of the battery car will be significantly reduced, which is not only suitable for girls, middle-aged and elderly people, but will also significantly improve car safety after weight reduction.
The major manufacturers of lithium-ion batteries are Sanyo, Sony, Samsung, and Matsushita. These four companies have a market share of more than 50%. Lithium-ion battery applications include mobile phones, notebook computers, photographic equipment, game consoles, etc., which began to be used in power tools in about 2005, and gradually used in electric vehicles after 2010. There are two types of battery structure, square and cylindrical, as shown in Figure 1.2. The cylinder has high mechanical strength, high energy density, and square heat dissipation. For the case where many single cells form a battery stack, this difference between the two will be amplified, involving the optimization of the structure of the battery stack.
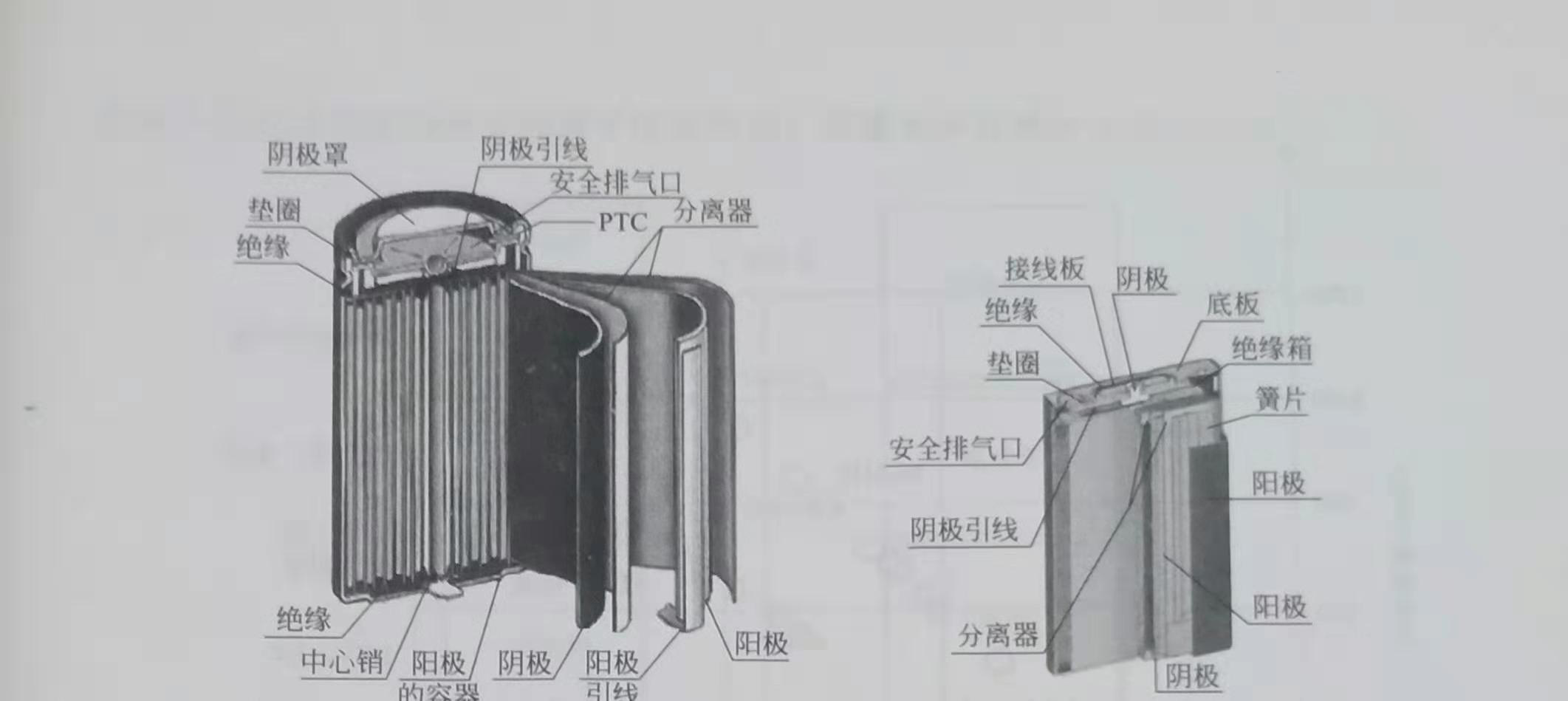
Figure 1.2 Wound cylindrical and square ion batteries
A typical 18650 cylindrical battery has a diameter of 18mm and a height of 650mm. Its capacity has evolved over time, as shown in Figure 1.3. In 2003, the energy density of the 2.4A.h battery was 200W · h / kg and 500W. h / L, currently about 250W · h / kg and 640W · h / L.Initially, hard carbon was used as the negative electrode material, with a capacity of 150 ~ 200mA · h / g. At that time, the electrolyte was poorly compatible with graphite materials. The capacity improvement was due to the following points:
① The development of new electrolyte additives has gradually applied high-capacity graphite anodes;
② The development of functional electrolyte allows to increase the upper charge limit of the positive electrode, which is equivalent to increasing the capacity of the positive electrode;
③ The emergence of new high-capacity cathodes, for example, the ternary layered material with higher capacity than lithium diamond acid, the emergence of lithium-rich manganese-based solid solutions. At the same time, the capacity is increased at the same time, and the energy is slightly reduced.
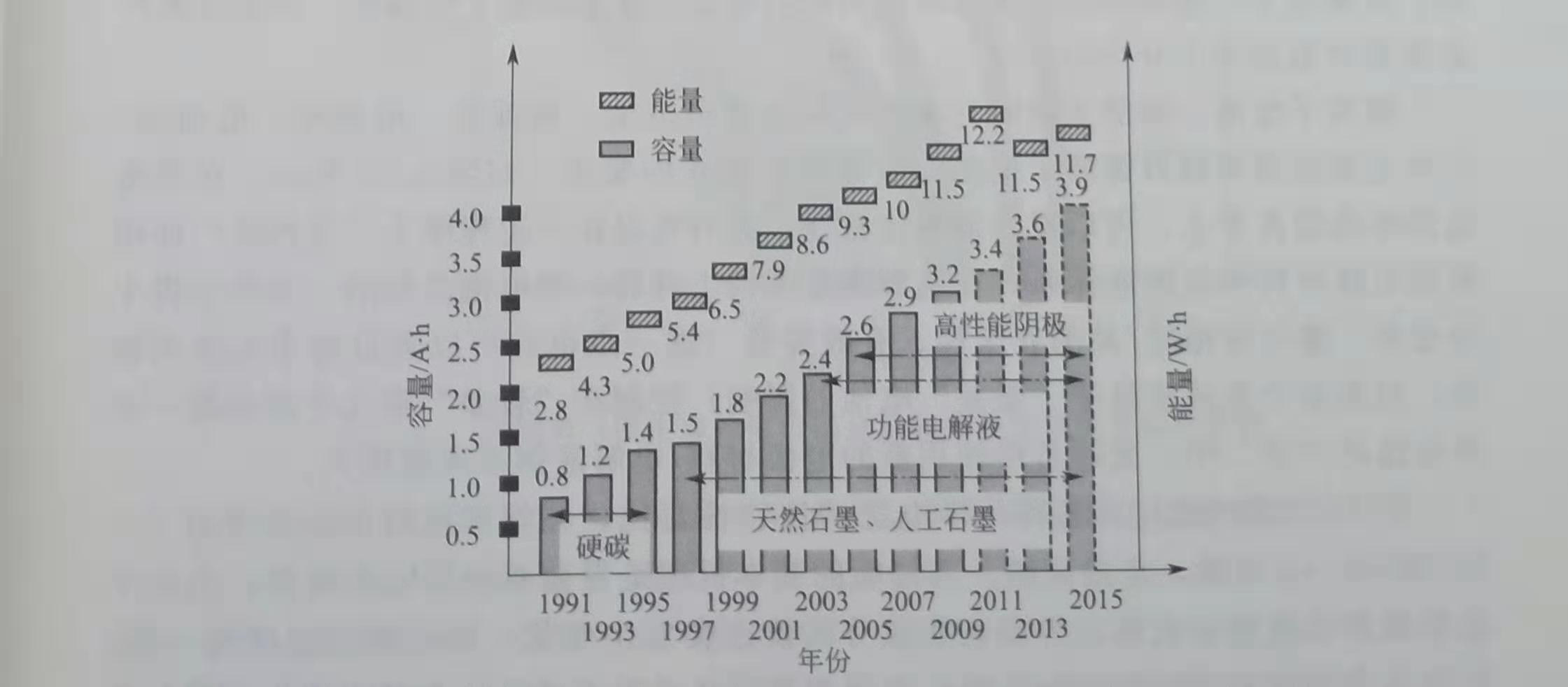
Figure 1.3 Capacity and energy evolution of 18650 cylindrical battery
-
skype
Zale Zhou
