LiNiPO4
2020-02-06 14:10:56
At room temperature, LiNiPO4 appears to be insulating. As the temperature increases, the electrical conductivity gradually improves, 10-14S / cm at 100 ° C, 10-12S / cm at 200 ° C, and 10-11S / cm at 300 ° C, 400. 10-8S / cm at ℃. M. Prabu et al. Studied the lithium ion diffusion performance of LiNiPO4 material, and its Li diffusion coefficient was (2.7 ± 0.4) × 10-10cm2 / s. Secondly, the reversibility of LiNiPO4 materials is very poor. One possible reason is the collapse of the crystal lattice.
LiNiPO4 is an olivine-type structure with a hexagonal close-packed structure and belongs to the Pmna space group. Among them, the oxygen atoms are respectively combined with Li and Ni2 , occupying 1/2 of the octahedral position, and P5 occupying 1/8 of the tetrahedral position. The LiNiPO4 structure is chained in the c-axis parallel direction. A NiO6 octahedron coexists with two Li06 octahedrons and a PO4 tetrahedron, and a PO4 tetrahedron with one NiO6 octahedron and two Li06 octahedra on its sides, thereby forming a three-dimensional network。 Like structure. The PO4 tetrahedron between the octahedra restricts the change of the character volume, which affects the intercalation and disassembly of Li. In this phosphorus-containing micropetite structure, all the oxygen ions and P form a polyanionic POF (tetrahedron) with a strong covalent bond, which makes the overall spatial structure stable. In addition to the olivine-type structure, by changing the external environmental pressure, LiNiPO4 material with a sharp stone structure can be synthesized at 20 GPa, and a new structure can be generated at a pressure between 4 and 20 GPa, that is, metastable Na2CrO4 LiNiPO4 material, but the LiNPO4 cathode material of this structure has a different structure from the LiNiPO4 material of micropetite structure. Its Li independently occupies a tetrahedral position, hinders the diffusion of Li , and makes its electrochemical activity lower.
Lucangelo Dimesso and others believe that there are two phases in the LiNiPO4 cathode material during the delithiation process, that is, LiNiPO4 and NiPO4, or a combination of the two phases. Manickam Minakshi et al. Thought that there were irregular NiPO4 formation during the lithium ion de-intercalation process. At present, there are not many studies on the lithium ion deintercalation mechanism of LiNiPO4 cathode materials, because the pure phase LINiPO4 material has low conductivity and high voltage, which makes it difficult to perform charge and discharge tests.
The XRD test of samples obtained by MPrabu et al. Using the Pechini-type polymerizable precursor method and sintering at 800 ° C for 8 hours showed that they belong to the Pnma space group, and the unit cell parameters are: a = 1.007nm, b = 0.5860nm, c = 0.4620nm, V = 0.2726nm3. The Raman test shows that there is:-a strong absorption peak at 945cm, which is a tetrahedron (PO4) 3-a normal symmetrical vibration, at higher frequencies (1010cm-1, 1070cm ↓ and 1083cm-1) is (PO4) 3- The asymmetrical stretching vibration of 200 to 300cm1 is the asymmetric stretching vibration of Li-O bond.
Lucangelo Dimesso et al. Used the Pechini-assisted sol-gel method to synthesize LiNiyPO4. The CV curve shows that, as shown in Figure 1, when y = 1.0, the oxidation peak is 4.88V, and when y = 0.9 or 0.8, the oxidation peak is 5.17V, 0.1C. At y = 1.0, 0.9 and 0.8, the discharge capacity is 86mA.h / g. 76mA.h / g and 122mA.h / g; when y = 0.8, Li2Ni3 (P202) 2 impurities are present in the material.
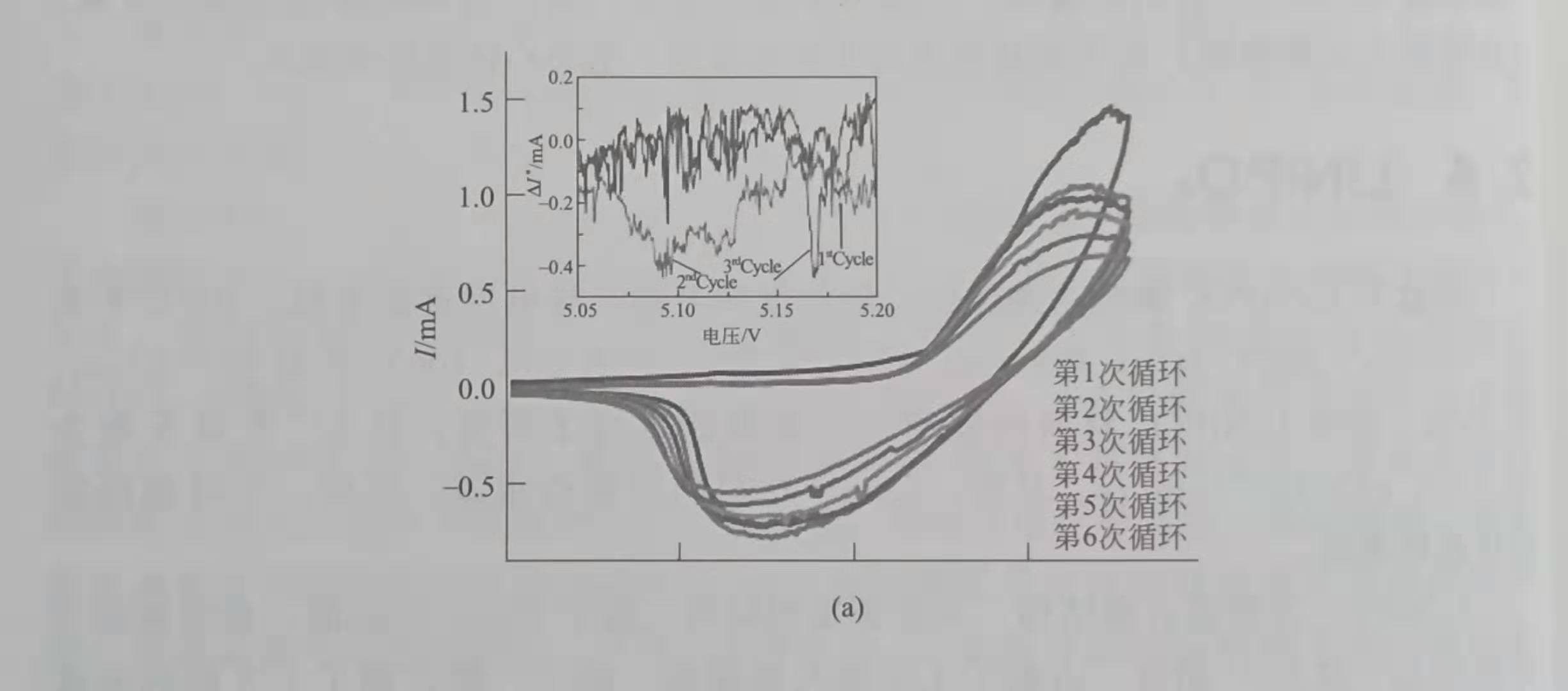
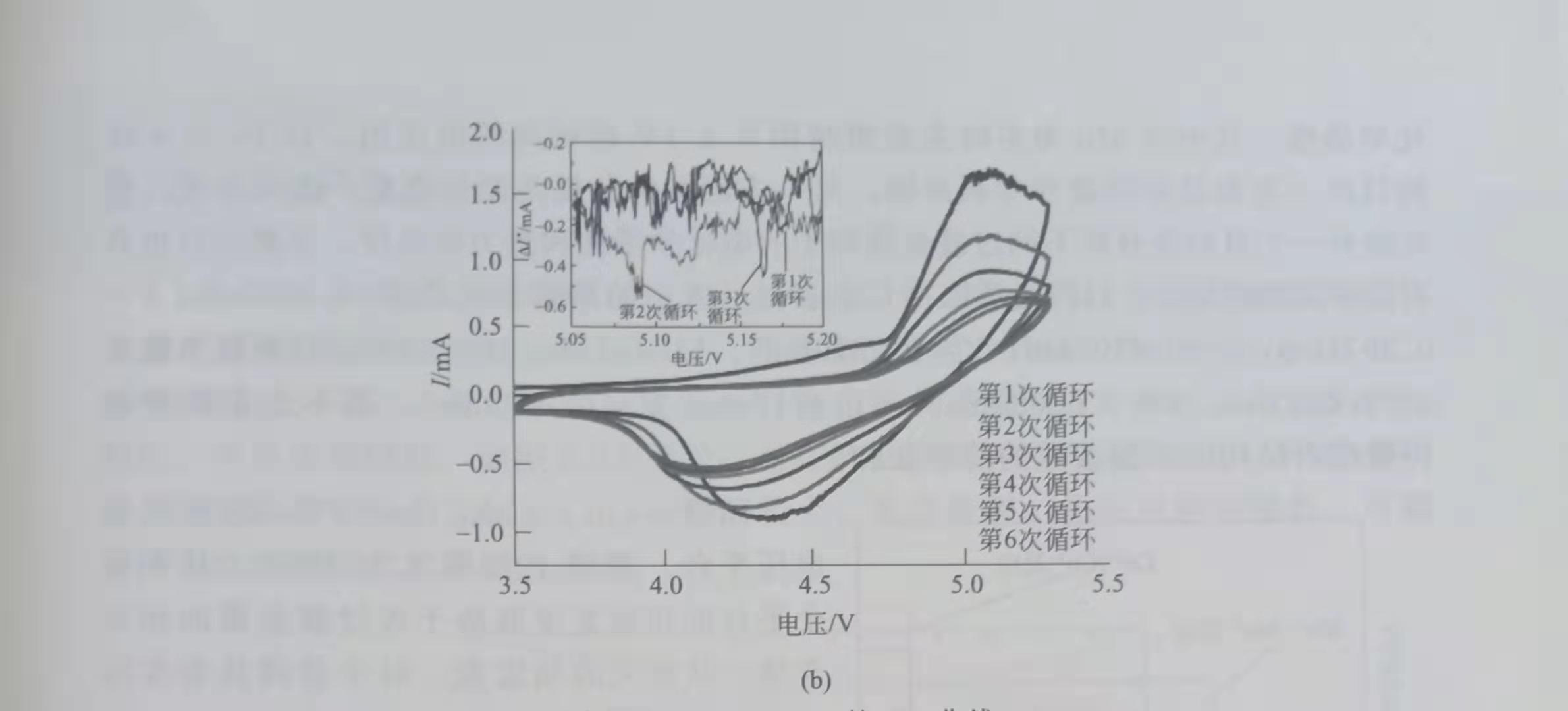
Figure 1 CV curve of LiNiyPO4
a) y = 0.9; (b) y = 0.8, scanning speed 0.2mV / s
LiNiPO4 with olivine-like structure, containing Ni3P and Li4P2O7 impurities, 1mol / L LiFAP / EC DMC electrolyte, 5.1 ~ 5.3V oxidation peak, 4.9V reduction peak on CV curve, 0.1C capacity is 86mA.h / g The capacity of LiNiPO4 reported by L Dimesso et al. Is close to 120mA at 0.1C. h / g.
The purpose of doping is to form inter-lattice defects or lattice expansion, improve the conductivity of LiNiPO4 materials, some can increase the diffusion rate of Li , and some can increase the electrochemical activity of the material. The substituted ions can be transition metals (Fe, Mn, Co) or non-electrochemically active metals (Mg, Ca, Al, Zn). The capacity does not increase after doping, but its structural properties and charge density can be changed. To improve its electrochemical performance.
-
skype
Zale Zhou
